Abstract
Background: Patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT) are at increased risk of infection-related mortality during the pre-engraftment period. Systemic antibiotic prophylaxis (SAP) is widely used during the period of neutropenia but is associated with disruption of the intestinal microbiome, an increased risk of Clostridium difficile infection (CDI) and colonization with multi-drug resistant (MDR) bacteria.
Aims: We retrospectively assessed the safety and efficacy of a change in our center policy from SAP to an exclusively interventional antibiotic treatment (IAT) in adult patients undergoing allogeneic HSCT. Outcome measures were overall survival (OS) and non-relapse mortality (NRM) at 3 years, antibiotic administration, incidence of blood-stream infections (BSI) and incidence and severity of acute and chronic graft-versus-host disease (GVHD).
Methods: Cefotaxime was used as standard of care prophylaxis during neutropenia after HSCT prior to January 2018. After a 12 months transition time during which both strategies were employed in parallel, SAP was replaced by an IAT strategy with meropenem being administered immediately by nursing staff in case of neutropenic fever or any other signs of infection, and subsequently deescalated to piperacillin/tazobactam (pip/taz) when appropriate. We compared 67 consecutive patients undergoing HSCT in 2017, all of whom received antibiotic prophylaxis (SAP cohort) with all patients in 2019 comprising the IAT cohort (n=68). Use of antibiotics is reported as number of daily doses per 100 care days (DDD/100CD) until discharge after HSCT.
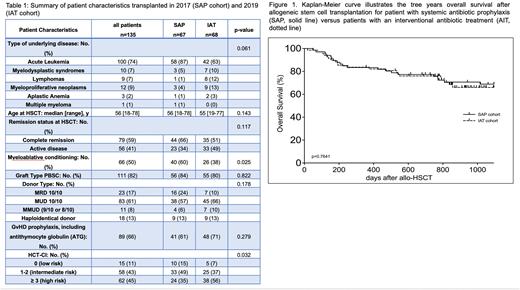
Results: Patient characteristics are summarized in table 1. Median age was 56 years, 82% of patients were grafted with peripheral blood stem cells (PBSC) for acute leukemia (74%) from a matched unrelated or related donor (MUD, 61%; MRD 17%). Patient characteristics were well balanced between the SAP and IAT cohorts, except those patients transplanted in 2019 were more likely to have an HCT-CI ≥ 3 (56% vs 36%, p=0.025) and receive reduced-intensity conditioning (62% vs 40%, p=0.025). In the SAP and IAT cohort, neutropenic fever was observed in 46% and 62% of patients (p=0.085). However, the cumulative incidence (CI) of BSI by day +100 post-HSCT was significantly higher in the IAT vs SAP cohort (40% vs 13%, p<0.001), which did not result in more patients admitted to the intensive care unit (13% vs 6%, p=ns) and did not translate into a higher CI of NRM at 3 years (10% vs 9%, p=ns). With a median follow-up of 1052 days, 3-year OS was 70% and 69% for the SAP and IAT cohort, resp. (p=ns, figure 1). CI of acute GVHD grade II-IV or chronic GVHD of any grade at 3 years was 34% (p=ns) and 45% (p=ns), resp., resulting in identical survival free of acute GVHD grade III-IV, chronic GVHD requiring systemic immunosuppressive therapy and relapse-free survival (GRFS) of 36% (p=ns) in both groups. We observed a tendency to a higher CI of severe chronic GVHD in the SAP cohort (27% vs 13%, p=0.08). There was no significant difference between the two cohorts in terms of newly detected colonization with MDR bacteria during conditioning and after HSCT (VRE 17%; ESBL 5%; 3/4 MRGN 5%; MRSA 0%) and the incidence of CDI was likewise equal in both cohorts (13%, p=ns).
Compared to SAP, IAT resulted in significantly fewer days of antibiotic therapy (24 vs 18, p<0.001) and accordingly less administration of cefotaxime (12.8 vs 3.8 DDD/100CD), teicoplanin (26.2 vs 6.3 DDD/100CD), vancomycin (11.5 vs 8.3 DDD/100CD) and meropenem (23.1 vs 16.7 DDD/100CD). Solely use of pip/taz increased (14.3 vs 23.6 DDD/100CD), while the amount of imipenem remained unchanged (7.6 versus 7.8 DDD/100CD). Thus, we observed a significant 40% reduction of the median antibiotic consumption (p=0.0312) in 2019.
In the multivariate analysis, only age > 60 years and a high or very high disease risk index were identified as negative prognostic factor for OS.
Conclusion/Summary: Our single center experience in conducting HSCT without antibiotic prophylaxis but with strict guidelines for prompt antibiotic intervention demonstrated no disadvantage in OS and NRM, despite a higher CI of BSI. IAT resulted in considerably less consumption of cefotaxime, but also carbapenem and glycopeptide antibiotics. We conclude that the proposed procedure of IAT replacing SAP is safe and feasible. Larger patient numbers are needed to confirm a possible benefit of IAT on GVHD.
Disclosures
Bug:Pfizer: Consultancy; Jazz: Honoraria; Celgene /BMS: Consultancy, Honoraria; Novartis: Consultancy; Gilead: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal